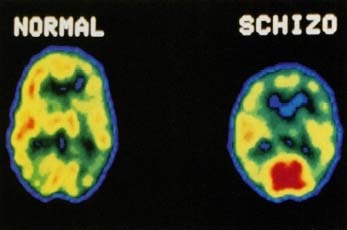
Pomaglumetad Methionil (mGlu2/3) is a glutamatergic-based agent that, unlike all currently available antipsychotic drugs, does not interact with those central nervous system receptors that are thought to be responsible for many of the intolerable adverse events (e.g., motor dysfunction, reproductive hormone irregularity, weight gain, lipid elevation) associated with present schizophrenia treatments.
The company has said, in the study mGlu2/3 did not separate from placebo in the primary efficacy endpoint at the two doses investigated (40 mg and 80 mg BID). Although the active control –risperidona- did separate from placebo in both populations. This trial is the first of two experiments that would support registration pomaglumetad methionil to treat acrute schizophrenia.
“Unfortunately negative studies are common in the field of psychiatry and a reality of biopharmaceutical innovation”, said Jan Lundberg, Ph.D., Executive Vice President, Science and Technology, and President, Lilly Research Laboratories. Despite all, the second clinical trial (HBBN) is going ahead and the company expects to get positive results at the end of the year.
Lilly is also awaiting results from a recently concluded study exploring the use of the drug as an adjunctive treatment with atypical antipsychotics. Data from these two studies will help Lilly to make decisions on the future development of pomaglumetad methionil.

No comments:
Post a Comment